
PCV2d is now the predominant genotype in Korea [1] and has been previously isolated in PCVAD cases in vaccinated herds [2]. As PCV2 vaccines were initially produced from PCV2a genotype, this study was performed to compare efficacy of a recently registered vaccine from PCV2d genotype with a vaccine from PCV2a genotype according to a challenge by a PCV2d strain isolated from an outbreak of PCVAD in Korea.
Materials and methods
Eighty colostrum-fed conventional piglets were randomly allocated to 4 groups of 20 each. They were negative against PCV2, PRRS and M. hyopneumoniae (Elisa and PCR tests). Groups T and C were vaccinated with PCV2 vaccines (respectively Suigen® PCV2, Virbac and Ingelvac CircoFLEX®, Boehringer Ingelheim) at 3 weeks of age (1 ml/pig by IM route).
UnVac/Ch and UnVac/UnCh groups were injected with 1 ml/pig of PBS by IM route. Groups T, C and UnVac/Ch were inoculated intranasally by 2 ml (1 ml/nostril/pig) inoculum containing 105TCID50/ml of a PCV2d strain at 7 weeks of age.
UnVac/UnCh group was inoculated by 2 ml (1 ml/nostril/pig) of uninfected cell culture supernatant. Blood samples were taken on all pigs every 2 weeks between vaccination and challenge, then weekly between challenge and necropsy on 28 days after challenge. Serum PCV2d genomic copies (by qPCR), serum PCV2d neutralizing antibodies (NA)
titer, number of PCV2d interferon- γ secreting cells (IFN- γ-SC) in peripheral blood mononuclear cells (PBMC) and number of PCV2 infected cells in superficial inguinal lymph node (by immunohistochemistry: IHC) were quantified by previously published methods. Data were analyzed by a general linear mixed statistical model.
Results
No side effects were reported in vaccinated groups. NA titers and IFN-γ-SC were detected in vaccinated groups from 14 days after vaccination and peaked respectively 21 and 14 days after challenge.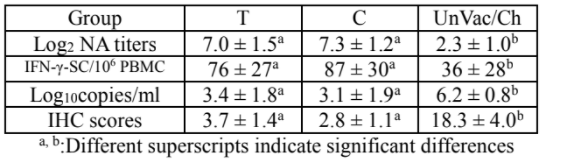
The mean NA titers and IFN-γ-SC were significantly higher in vaccinated groups than in UnVac/Ch group between challenge and 28 days after challenge. Viremia peaked on 21 days after challenge.
The mean serum genomic copies of PCV2d were significantly lower in vaccinated groups than in UnVac/Ch group between 2 and 4 weeks after challenge. The mean lymph node IHC scores were significantly lower in vaccinated than in UnVac/Ch group. The above criteria remained below limits of quantification in UnVac/UnCh group during the whole study.
Conclusion
Both vaccines were effective in inducing specific immunity against PCV2d and reducing blood and tissue viral burdens, thus confirming cross protection between genotypes. No significant difference was found between vaccines
according to the challenge model tested. In a more severe model combining PCV2 and PRRS infections, viremia was better controlled to some extent with homologous genotype between vaccine and challenge [3]. Thus PCV2a and d vaccines could be further investigated in a model combining PCV2d and PRSS infection, as combination of both viruses induces more severe disease [4].
Acknowledgement: The author’s research was supported by contract research funds from Virbac (Grant no. 550-20180009).
References:
[1] Seung-Chai K et al., 2018. BMC Vet Res 14:294. [2] Seo HW et al., 2014. Arch Virol 159, 3107–3111. [3] Opriessnig T et al., 2014. Vaccine 32, 230-237. [4] Park C et al., 2014. J Gen Virol 95, 2486-2494.
